Functional Materials
Photocatalytic TiO2
Photocatalytic Titanium Dioxide, ST/STS Series
Titanium Dioxide essentially exhibits strong photo activated oxidization when irradiated by UV ray.
The pigmented titanium dioxide for paints and plastics usually suppress the photo activity with the surface treatment. Photocatalytic titanium dioxide maximizes the essential photo activity.
Photo catalyst decomposes the organic pollutants and malodors into carbon dioxide and water by the photo activated oxidation. And it presents antiviral or antibacterial activity. It also induce the high water affinity by photo activated super-hydrophilicity, which generates anti-fogging, anti-stain, self-cleaning performance. Furthermore, the photo activated reaction is sustained as a regenerative action.
ISK's photocatalytic titanium dioxide ST, STS products was developed with our unique technology to strengthen the photo activity.
Strengths
-
Strength 1Diverse product portfolio (powders, water dispersions, and coating materials)
-
Strength 2Functionality added by surface treatment
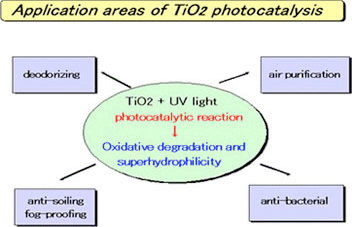
Typical Applications
- Deodorization
- Air purification
- Anti-fogging
- Antibacterial/antiviral activity
Visible Light Photocatalyst:MPT-623, STS-427
ISK has intended to use the photocatalyst indoors, and developed a photocatalytic powder, which can be activated by visible light like fluorescence light. (Developmental product: MPT-623). It is expected to perform the decomposition of malodors, antiviral and antibacterial activity indoors.
Antiviral activity test (BacteriophargeQβ)
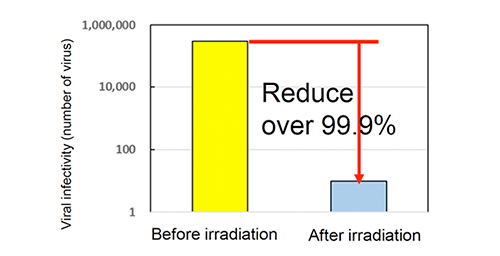
【Test method】
Antiviral activity test (Photo catalyst)
Bacteriopharge(JIS R 1756)
Fluorescent lamp 500 lx, 4 hours
Sharp Cut Filter TypeB
Cut under 380nm
Photocatalyst: 0.15 g/m2
Antibacterial activity test (Staphylococcus aureus )
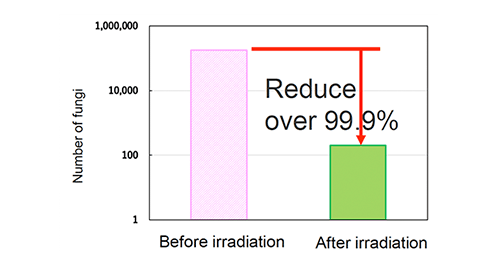
【Test method】
Antibacterial activity test (Photo cataylst)
Staphylococcus (JIS R 1752)
Fluorescent lamp 500 lx, 4 hours
Sharp Cut Filter TypeB
Cut under 380nm
Photocatalyst: 3.6 g/m2
※Aqueous dispersion STS-427 is also available.
UV Light Photocatalyst
UV Light Photocatalyst exhibits high photo activity, when it is used exterior under substantial UV light of Sunlight. It can be applied for the filter of air cleaner in combination with UV lamp.
● Photocatalytic Powder ST Series
1. Characteristics of ST series
Various grades with different particle size and adsorption properties are available.
*You can see the entire image in the figure by scrolling horizontally.
| Grade | X-ray diameter(nm) | SSA(m2/g) | Photoactivity | Characteristics |
|---|---|---|---|---|
| ST-01 | 7 | 300 | ◎ | highest photoactivity |
| ST-21 | 20 | 50 | ○ | high photoactivity and dispersibility |
| ST-31 | 7 | 250 | ○ | excellent gas absorption |
| ST-41 | 200 | 10 | △ | excellent dispersibility |
2. Photocatalytic properties of ST series
・Gas phase (acetaldehyde)
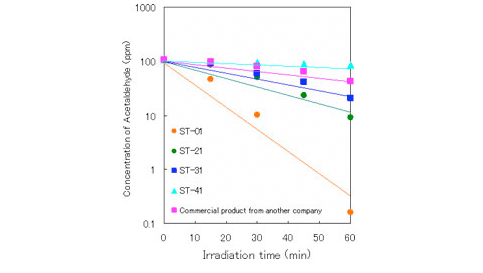
(Condition)
amount of TiO2: 0.1g
light source intensity: black light 0.5mW/cm2
irradiation area: 24cm2
reaction volume: 2.8L
・Liquid phase (2-propanol)
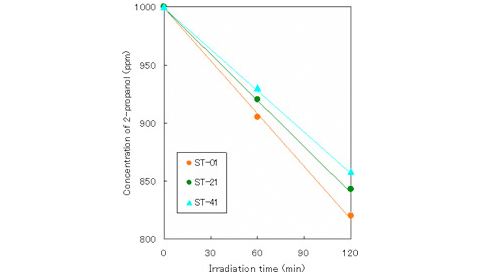
(Condition)
amount of TiO2: 4g/L
light source intensity: black light 2mW/cm2
irradiation area: 24cm2
reaction volume: 80mL
● Photocatalytic Hydrosol STS Series
Acidic and neutral sols are available.
*You can see the entire image in the figure by scrolling horizontally.
| Grade | TiO2content (wt%) | X-ray particle size (nm) | pH | Characteristics |
|---|---|---|---|---|
| STS-01 | 30 | 7 | 1.5 | acidic with nitric acid, excellent dispersibility |
| STS-02 | 30 | 7 | 1.5 | acidic with hydrochloric acid, excellent dispersibility |
| STS-21 | 40 | 20 | 8.5 | slightly basic and easy to handle |
● Photocatalytic titanium dioxide coating agents ST-K series
The ST-K series is a group of photocatalytic coating agents that uses an inorganic binder.
Since a highly transparent coating film is produced, you can expect it to have antifouling and antibacterial effects on the surface of aesthetically appealing substrates.
1. ST-K200 series (top coat agents)
The ST-K200 series comprises super-hydrophilic coating agents made from photocatalytic titanium dioxide. Because it uses a sol of ultrafine titania particles, a highly hard and transparent coating can be obtained.
The surface of the coating becomes hydrophilic due to the ultraviolet rays contained in sunlight, producing effects such as prevention of droplet formation, anti-fog property, self-cleaning property, and ease of cleaning.
*You can see the entire image in the figure by scrolling horizontally.
| Grade | Solid content (wt%) | Appearance | Viscosity (mPa∙s) |
Solvent | Characteristics | Effects |
|---|---|---|---|---|---|---|
| ST-K211 | 0.2 | Milky white | 1 to 5 | Alcohol/Water | Baking at any room temperature Up to 500°C | Self-cleaning, anti-fogging, prevention of droplet formation, and ease of cleaning |
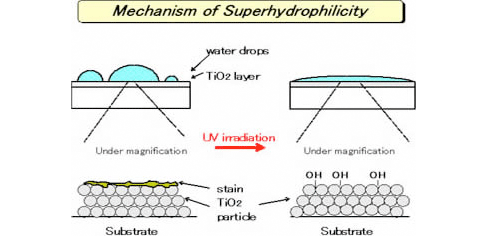
2. Photo-induced super-hydrophilicity
The surface coated with photocatalytic TiO2 shows super-hydrophilicity by UV irradiation. On the super-hydrophilic surface, adsorbed water does not form water drops but become thin layer. Thus, the surface exhibits anti-fogging effect and prevent the stain on the surface. Furthermore, the surface becomes easy to wash away a stain by water even if it adhered.
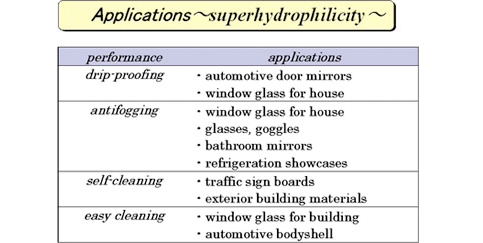
This phenomenon is called “photo-induced super-hydrophilicity” and draws an attention as new feature of a photocatalyst. It is used for various applications as below.
3. Decomposition of organic dyes (ST-K211 coated on a glass substrate and UV-irradiated)


