Business Introduction
Pharmaceuticals, animal health products
Linking sophisticated organic synthesis technology with our lives
ISK’s chemical business also covers medical care.
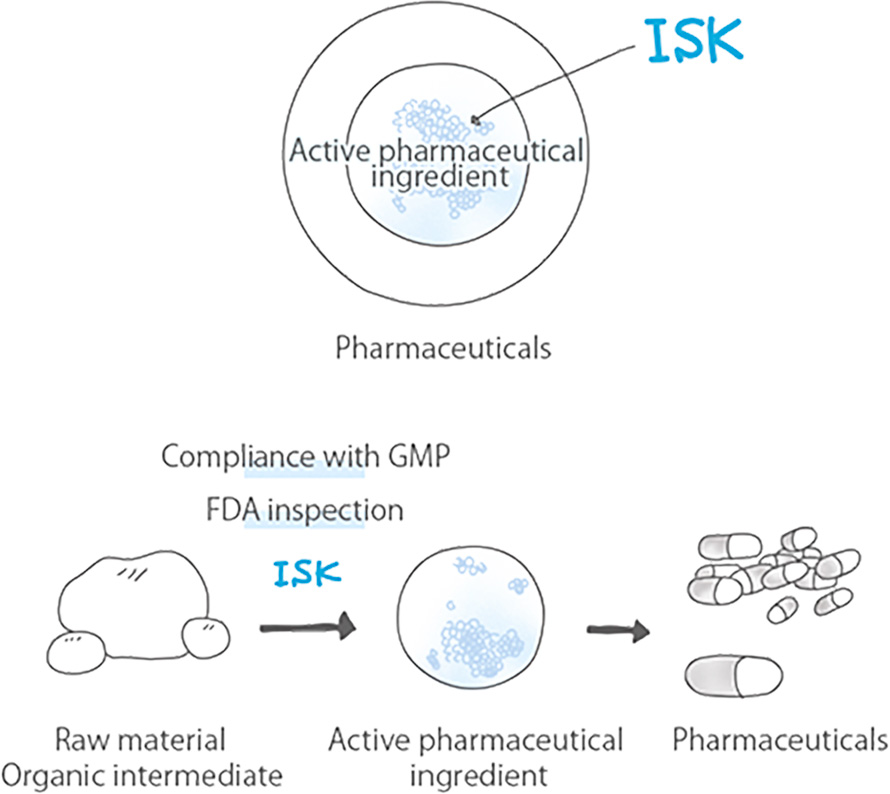
ISK applies the organic synthesis technology it has cultivated through its agrochemical business to the development and production of pharmaceuticals.
In 1998, we completed a plant that complies with the Good Manufacturing Practice (GMP) standard for manufacturing pharmaceutical products. We passed inspections conducted by the U.S. Food and Drug Administration (FDA) and gained manufacturing approval. Since 2001, we have manufactured the active pharmaceutical ingredient Cevimeline Hydrochloride Hydrate, a drug to treat Sjogren’s syndrome, where symptoms such as dry mouth occur due to salivary and lacrimal gland disorders, and have started supplying it to domestic pharmaceutical companies.
The GMP factory in our Yokkaichi Plant is used exclusively to produce pharmaceuticals. This factory is equipped with a clean room that can maintain the air cleanliness at the same level as that used to fabricate precision machines (class: 100,000), a chromatography column for the final purification in a multistep synthesis, a filtration/drying process, pulverization/mixing process, and a system for purifying water in compliance with the USP purified water standard (completely impurity-free water). This factory is also equipped with highly reliable effluent and waste treatment systems.
Pharmaceuticals pass through many synthetic processes until they are completed as products. ISK produces raw materials of pharmaceuticals called organic intermediates. These intermediates are derived from the synthetic process. In particular, the development of a CTF (a CF-3 pyridine derivative) production process, which won the 36th Okochi Award in 1981, created an opportunity for our technology to be reputed highly both inside and outside Japan.
ISK’s chemical products for the health of familiar animals.

Companion animals are kept as a family’s important members. Only people can protect the invaluable lives of familiar animals.
However, there are many disease areas that are not covered by veterinary drugs, and more than 80% of the drugs used in Japan must be diverted from human drugs, while taking into account the risks. The reason behind this is that there are still very few therapeutic drugs developed specifically for companion animals.
In the field of veterinary medicine, ISK will direct its resources to R&D in areas where therapeutic drugs are awaited due to the absence of medical treatments and where veterinarians need new drugs. As a first step, for the anti-inflammatory drug for the acute phase of pancreatitis in dogs that was developed using fuzapladib sodium hydrate, we obtained approval to manufacture and sell the product BRENDATM in Japan in 2018, followed by the approval for PANOQUELLTM in 2024. Additionally, we obtained conditional approval to manufacture and sell the product PANOQUELLTM-CA1 in the U.S. in 2022. ISK’s chemical business will continue to support the health of animals by applying basic technologies cultivated through agrochemical business.
