Animal Health Products
Taking advantage of our new pesticide discovery technology that we have fostered so far, we have developed (fuzapladib), an anti-pancreatitis drug for dogs.
We provide excellent products that the owners of companion animals and animal healthcare professionals depend on.
Strengths
-
Strength 1Development, manufacture, and sales that integrate the various functions of R&D, marketing, and pharmaceutical regulatory affairs management
-
Strength 2Access to a global market centered on the U.S. and Europe
-
Strength 3Able to develop related products for product lifecycle management; a wealth of seed ideas for new product developmen
Typical Applications
- Anti-inflammatory drug for acute phase of pancreatitis in dogs
Fuzapladib Sodium Hydrate
In September 2018, we obtained manufacturing/marketing approval for this drug with the effect of “improvement of clinical signs in the acute phase of pancreatitis in dogs”.
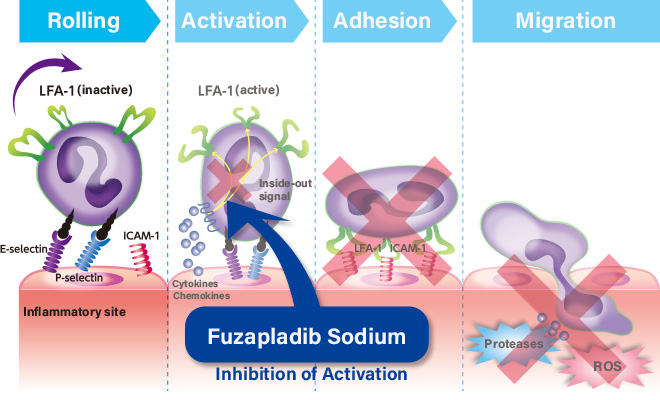
Fuzapladib blocks activation of adhesion molecules (integrin) expressed on the inflammatory cell surface to prevent inflammatory cells from adhering to vascular endothelial cells and infiltrating tissue and to control exacerbation of pancreatitis.
This mode of action is different from that of conventional anti-inflammatory drugs including steroids and non-steroid anti-inflammatory drugs (NSAIDs).
PanoquellTM-CA1, which uses this active ingredient, received conditional approval from the FDA in November 2022. Its sales in the United States began in April 2023.
The newly launched “PanoquellTM” is a product with the same composition as “PanoquellTM-CA1,” which is sold in the United States.
Furthermore, through our partnership with Ceva, a world-leading veterinary pharmaceutical company, we aim to promote the Panoquell brand throughout the world, including Europe.
Conditional approval from the FDA means that, when used according to the label, the drug is safe and has a reasonable expectation of effectiveness. Veterinarian access to critical animal drugs through conditional approval provides options for treating animals with uncommon conditions, serious or life-threatening diseases, or diseases without existing or adequate therapies.
