Environmental Products
Combined Solution
Combining our environmental products (GypsanderTM, Fix-AllTM, and MT-V3TM) will enable you to take advantage of the characteristics of each to address particular contaminated soil problems.
Strengths
-
Strength 1Combination technology to address particular contaminated soil problems
Typical Applications
- GypsanderTM + Fix-AllTM
⇒ Solidification and insolubilization of heavy metals - GypsanderTM + MT-V3TM
⇒ Solidification and VOC decomposition - Fix-AllTM + MT-V3TM
⇒ Insolubilization of heavy metals including hexavalent selenium
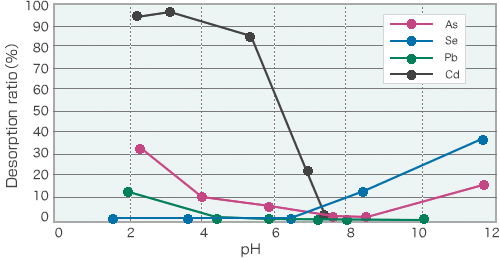
GypsanderTM + Fix-AllTM
In the absorption and insolubilization of heavy-metals-polluted soil, when cement-based solidifiers are used to harden adjusted soil, the soil tends to become alkalized, resulting in possible re-migration of arsenic and selenium. To avoid these phenomena, neutral solidifiers are recommended.
pH desorption curve of iron oxide-based absorbers
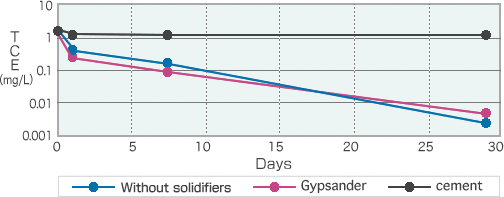
GypsanderTM + MT-V3TM
When carrying out decomposition and solidification simultaneously, the decompositional performance of soil adjusted using GypsanderTM remains the same as when done without solidifiers, but soil adjusted using cements is hardly decomposed.
Polluted soil tends to be soft, with solidification commonly required in the cleanup process.
When decomposing and cleaning up VOCs simultaneously with solidification, neutral solidifiers are recommended, so as not to compromise decompositional performance.
Decompositional performance of TCE
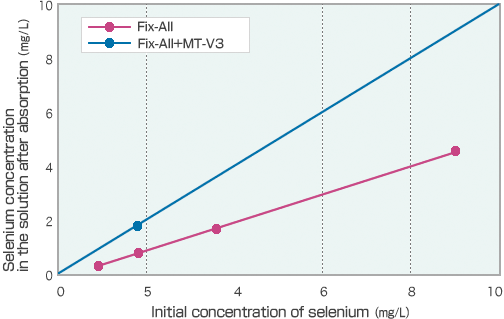
Fix-AllTM + MT-V3TM
When insolubilizing selenium, its valence is largely affected, where tetravalent selenium is insolubilized solely by Fix-AllTM, while hexavalent selenium is hardly insolubilized.
Combining Fix-AllTM with MT-V3TM enables selenium absorption to Fix-AllTM, as MT-V3TM is thought to act as a reduction agent in converting hexavalent selenium to tetravalent selenium.
Comparing absorption performance of hexavalent selenium